Science
Interesting Facts about Mole you need to know on Mole Day
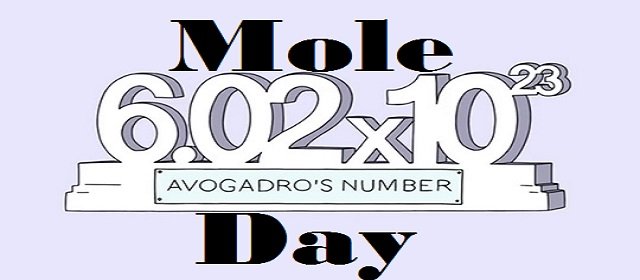
Mole Day is celebrated every year on October 23. The day this year will be seen from 6:02 a.m. to 6:02 p.m. The day is well known among chemists, chemistry students, and devotees. The time duration of Mole Day recognizes Avogadro’s Number, which is a basic measuring unit in chemistry. Avogadro number is denoted as 6.02×1023.
One mole is a mass (in grams), the number of which equals the molar mass of a molecule. Mole is one of all the seven base SI units. The day was brought into being to start enthusiasm for the subject of Chemistry. Numerous schools across the United States observe Mole Day.
What is a mole?
For a given molecule, one mole is a mass (in grams) whose number is equivalent to the molar mass of the molecule. For better understanding let us take an example of a water molecule. It has a molar mass of 18 that is one mole of water weighs 18 grams. Essentially, one mole of neon has a molar mass of 20 grams. That is one mole of any substance contains Avagadro’s Number of molecules or atoms of that substance. Madeo Avogadro, for the first time, found this relationship and after his demise, he got the credit for this.
Mole Day History
Mole Day can be recognized as an event that individuals often celebrate because of their enthusiasm for specific things. The day started around the 1980s when an article showed up in The Science Teacher. Afterward, a retired high school chemistry teacher Maurice Oehler from Wisconsin, USA, drew motivation from the article and established the National Mole Day Foundation. Numerous secondary schools in the United States, South Africa, Australia, and Canada mark Mole Day after May 15, 1991, when the establishment was set up. The goal is to make students intrigued by science by coordinating a few activities related to moles and chemistry. The Foundation became a not-for-profit corporation in 1992 in the State of Wisconsin.
The recently settled “National Mole of the Year” Award was first introduced at the ChemEd ’93 conference at Buttler University. This award proceeded with presentations at ChemEd ’95 at Old Dominion University and ChemEd ’97 at the University of Minnesota. Truly it is the “Mole-of-the-Every-Other Year” as the award is introduced in odd-numbered years at the ChemEd Conferences.
As the years have passed, enthusiasm within the National Mole Day Foundation has gotten far-reaching. The day is praised in different foreign countries, particularly in Canada and in Australia. In Australia, the CSIRO (which is comparable to NSTA) supports each educator of science subject to observe National Mole Day. In this way, we can say that NMDF was made with the expectation to create an interest in chemistry.
Mole Day 2020 Theme
The theme for Mole Day this year is MOLEzilla.
Mole Day Significance
The day is a unique day for all the people related to the subject of chemistry. Numerous activities identified with moles and chemistry are directed to mark this day. At the point when written in American format, the day takes after the Avogadro number which connotes the number of molecules or atoms contained in a mole. The American Chemical Society additionally supports the National Chemistry Week, on this event, for a week.
30 Interesting and Fun Facts about Mole
- Mole, likewise spelled mol, in chemistry, a standard scientific unit for measuring huge amounts of little substances, for example, atoms, molecules, or other indicated particles.
- The mole is credited to 18th century Italian researcher Amedeo Avogadro, whose full name is Lorenzo Romano Amedeo Carlo Avogadro di Queregna e di Cerreto.
- A mole is a unit of measurement used in chemistry and other related fields like biochemistry and biophysics.
- It is one of the perceived units of measurement in the International System of Units, identified with Avogadro’s Constant, and is represented by the abbreviation “mol.”
- Mole Day was proposed in an article in The Science Teacher in the mid-1980s. Inspired by the article, Maurice Oehler, a chemistry teacher (now retired) in Prairie du Chien, Wisconsin, made the National Mole Day Foundation in 1991.
- If there was a mole of rice grains, all the land regions in the entire world would be covered with rice to a depth of around 75 meters.
- One mole of rice grains is a greater number of grains than all the grain that has been developed since the get-go.
- One mole of rice would involve a cube around 120 miles on an edge.
- Computers can check at the rate of more than 800 million counts for every second. Along this rate, it would take a computer more than 25 million years to count to 6.02×1023.
- Avogadro’s Constant characterizes a mole as having a value of 6.02214129(27) x 1023, as it refers to the number of particles of a substance.
- The essential connection is that a mole is the amount of any known or unknown substance that has as many elementary entities as there are molecules in twelve grams of pure carbon-12.
- That implies that one mole of pure C-12 must have a mass of twelve grams all together for the unit of measurement to arrange.
- For quite a while, hydrogen and afterward oxygen-16 became the standard for correlation in measuring units, following the use of mass spectrometry.
- Wilhelm Ostwald coined the term mole in 1894, abbreviated from the German word for a molecule.
- The number of elementary entities in any substance is known as its chemical amount, which makes the mole a simple to use a unit of measurement for chemical amounts.
- A mole of marshmallows would cover the United States to a depth of 600 miles
- To place a mole of raindrops in a 30 meter (around 100 feet) diameter tank, the sides of the tank would need to be 280 times the distance from the Earth to the Sun.
- A mole of hockey pucks would be equivalent to the mass of the Moon.
- Assuming that every human has 60 trillion body cells (6.0 x 1013) and the Earth’s populace is 6 billion (6 x 109), the total number of living human body cells on the Earth right now is 3.6 x 1023 or somewhat over half of a mole.
- In chemistry, a mole is a more advantageous standard unit than endeavoring to quantify in mass or volume, particularly in chemical equations.
- The portrayal in measurement is additionally streamlined by the idea of the molecular mass, which expresses that the mass of one mole of a substance (measured in grams) is equivalent to the molecular mass.
- Consequently, one mole of a substance is equivalent to the molecular mass of a similar substance.
- The advancement of the mole as the standard unit of measurement for calculating the elementary entities contained within a substance has a history that goes back to the first table of relative atomic mass, created by John Dalton in 1805.
- Afterward, Jons Jacob Berzelius was instrumental in redefining relative atomic mass with a more prominent level of accuracy.
- If one mole of pennies were split among the Earth’s populace, every individual would get 1 x 6.02×1014 pennies. Individual spending at the rate of 1,000,000 dollars a day would go through every person’s wealth in around 3,000 years. Life would not be agreeable because the surface of the Earth would be covered in copper coins to a depth of at least 400 meters.
- If you had a mole of pennies and wanted to purchase kite string at the rate of 1,000,000 dollars for each inch, you would get your cash’s worth. After extending your string around the Earth one million times, and to the Moon and back 25 times, you would have enough string left over to sell back at a penny an inch (a decided loss) to increase enough cash to purchase each man, woman, and child in the US a $5000 automobile and enough gasoline to run it at 55 mph for a year. After those buys, you would at present have enough cash left over to give each man, woman, and child in the whole world $6136.
- There has been contention over the use of the mole as a standard unit of measurement since its proper reception in 1971, generally because researchers have since quite a while ago realized that knowing the mass of a substance isn’t generally vital for measurement.
- While the individuals who work in a chemistry field depend on the mole as a unit of measurement, its application for the industry is impractical since the amount is so little.
- The kilomole was acquainted with define larger sample units, which is the number of entities in twelve kilograms of carbon.
- Starting in 2011, the governing body over weights and measures consented to research options in contrast to the mole.
-
Health3 weeks ago
Back to Roots: Ayurveda Offers Natural Cure for Common Hair Woes
-

 Tech4 weeks ago
Tech4 weeks agoFrom Soil to Silicon: The Rise of Agriculture AI and Drone Innovations in 2025
-

 Science1 week ago
Science1 week agoJuly Full Moon 2025: Everything You Should Need to Know, When and Where to See Buck Moon
-

 Sports3 weeks ago
Sports3 weeks agoFIBA 3×3 World Cup 2025: Full Schedule, Preview, and How to Watch
-

 Gadget4 weeks ago
Gadget4 weeks agoThings to Know about Samsung Galaxy S26: What’s New and What’s Next
-

 Tech4 weeks ago
Tech4 weeks agoAdobe Firefly App Now Available on iOS and Android Phones to Create AI Images and Videos Anywhere
-

 Sports2 weeks ago
Sports2 weeks agoPrefontaine Classic 2025: Full Schedule, Preview, Field, Events and How to Watch Diamond League Eugene Live
-

 Festivals & Events4 weeks ago
Festivals & Events4 weeks agoEverything You Should Need to Know about Summer Solstice 2025













