World
Biden administration planning to share AstraZeneca’s Covid vaccine with Canada and Mexico
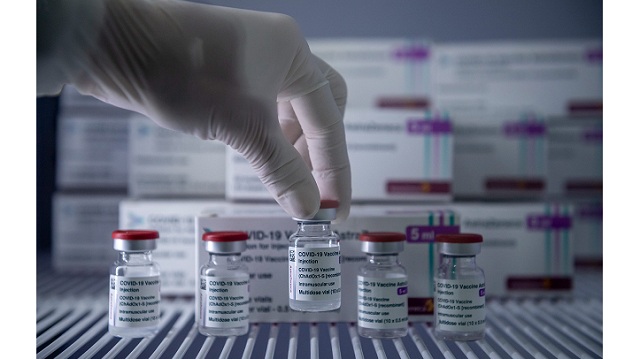
The Biden administration is concluding plans to share the U.S. supply of AstraZeneca’s Covid vaccine with the governments of Canada and Mexico, White House press secretary Jen Psaki affirmed Thursday.
During a March 1 bilateral meeting, President Joe Biden and Mexican President Andrés Manuel López Obrador discussed the likelihood that the U.S. would send Mexico overflow dosages of the AstraZeneca vaccine.
The subject of Mexico inclining up its law requirement presence at the U.S. southern line additionally came up, as indicated by an individual near the Mexican president and a senior U.S. administration official.
The Biden administration made a few references to the discussion soon after the official meeting. Yet, Psaki said definitively that same day that Biden would not consider Mexico’s public request that the U.S. government share its excess Covid vaccine because vaccinating Americans is a priority.
Psaki said Thursday that the White House is currently “assessing how we can loan doses” to the two nations bordering the U.S. “It’s not fully finalized yet. But that is our aim and what we’re working toward.”
The administration is planning to transport 2.5 million shots to Mexico and 1.5 million to Canada out of its 7 million “releasable dosages,” she added.
The Food and Drug Administration has thus far authorized crisis utilization of three separate vaccines for Americans — from drugmakers Pfizer, Moderna, and Johnson & Johnson — however, AstraZeneca has not yet filed for review.
When considered the frontrunner in the vaccine race, with guarantees that it could have dosages accessible by October 2020, AstraZeneca soon hit roadblocks with security concerns stopping preliminaries and afterward confusion over optimal doses slowing the rollout.
The organization’s vaccine rollout has likewise staggered as European nations that endorsed the shot before this year has pulled back proposals, refering to stress over blood clots. While the European Medicines Agency said Thursday that it actually believes the shot is protected and effective, certainty was shaken all through the bloc.
In any case, as AstraZeneca’s FDA review process proceeds, Psaki said the administration has been “taking action … to get ready to get those vaccines to the American people if they are approved.” The organization could file for crisis approval by April.
A senior administration official disclosed to POLITICO recently that “we continue to evaluate” the vaccine’s safety data and that sending dosages to different nations is balanced against ensuring there are sufficient vaccines for wide access in the U.S. this summer.
The Trump administration ordered 300 million doses of the AstraZeneca vaccine in May 2020, making it the biggest vaccine order by far at the time.
Yet, Pfizer and Moderna soon dominated the British drugmaker, leading the government to grow its order with those organizations. Biden has since gotten 300 million doses each from Pfizer and Moderna.
-

 Business5 days ago
Business5 days agoCorporate Social Responsibility in Action: Amerilodge’s Support of Health and Education Causes
-

 Tech2 weeks ago
Tech2 weeks agoAdobe Releases New AI-powered Video Editing Tools for Premiere and After Effects with Significant Motion Design Updates
-

 Book4 weeks ago
Book4 weeks agoAuthor, Fighter, Builder: How Alan Santana Uses His Life Story to Empower the Next Generation Through UNPROTECTED
-

 Business3 weeks ago
Business3 weeks agoSpartan Capital Publishes 2026 Economic Outlook, Highlighting Volatility, Resilience, and Emerging Opportunities
-

 Startup4 weeks ago
Startup4 weeks agoCraig Bonn’s Guide for Spotting a Winning Pre-IPO Early
-

 Tech3 weeks ago
Tech3 weeks agoGoogle Introduces New Updates to Its Veo AI Video Generation Tool
-

 Lifestyle4 weeks ago
Lifestyle4 weeks agoKent Strunk: The Lifelong Twins Fans Building Family Traditions One Game at a Time
-

 Business3 weeks ago
Business3 weeks agoJohn Dianastasis Elaborates on the Value of Discretion in High-Level Consulting and Public Affairs Work

















